Copper-promoted cyanoalkylation/ring-expansion of vinylcyclopropanes with α-C–H bonds in alkylnitriles toward 3,4-dihydronaphthalenes†
Abstract
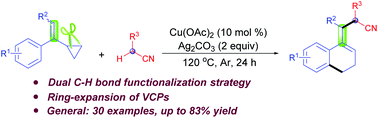
A copper-promoted oxidative cyanomethylation/ring-expansion of vinylcyclopropanes with α-C(sp3)–H bonds in alkyl nitriles is established for the generation of 1-cyanoethylated 3,4-dihydronaphthalenes. This cyanomethylation/ring-expansion involves a radical pathway and proceeds via cyanomethyl radical formation, radical addition and ring-expansion. This ring-expansion strategy offers a highly atom-economical route for the construction of nitrile-containing 3,4-dihydronaphthalenes, which can be transformed into other useful products under simple conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...