Mannich-type allylic C–H functionalization of enol silyl ethers under photoredox–thiol hybrid catalysis†
Abstract
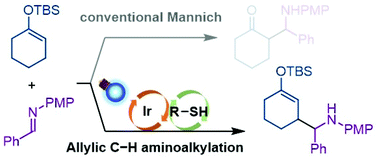
The synergy of an Ir-based photosensitizer with mild oxidizing ability and a thiol catalyst enables efficient allylic C–H functionalization of enol silyl ethers with imines under visible light irradiation. Subsequent transformations of the aminoalkylated enol silyl ethers allow for the facile construction of unique molecular frameworks such as functionalized octahydroisoindol-4-one.
- This article is part of the themed collections: Editor’s Collection, Hybrid Catalysis and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...