A medium-firm drug-candidate library of cryptand-like structures on T7 phage: design and selection of a strong binder for Hsp90†
Abstract
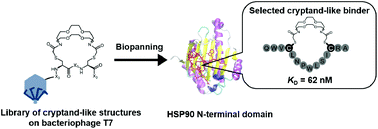
We designed and synthesized a medium-firm drug-candidate library of cryptand-like structures possessing a randomized peptide linker on the bacteriophage T7. From the macrocyclic library with a 109 diversity, we obtained a binder toward a cancer-related protein (Hsp90) with an antibody-like strong affinity (KD = 62 nM) and the binding was driven by the enthalpy. The selected supramolecular ligand inhibited Hsp90 activity by site-specific binding outside of the well-known ATP-binding pocket on the N-terminal domain (NTD).
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...