Unusual bridged angucyclinones and potent anticancer compounds from Streptomyces bulli GJA1†
Abstract
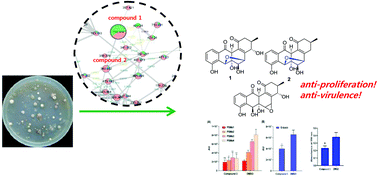
Two new unique angucyclinones (1 and 2) with unprecedented ether bridges connecting carbons 5 and 7 were isolated from the cultures of Streptomyces bulli GJA1, an endophyte of Gardenia jasminoides, together with two known ones (3 and 4). The MS2-based molecular networking system facilitated the isolation of compounds with target functionalities. The stereochemistry of 1 was completely established by ROESY and ECD experiments. Compound 3 showed antivirulence activities by inhibiting the peptide toxin (PSMs) production and the biofilm formation of MRSA. Compounds 3 and 4 showed very potent anti-proliferative effects against OV1 and ES2 ovarian cancer cells.


 Please wait while we load your content...
Please wait while we load your content...