Regioselective synthesis of tetrahydroquinolines via syn- and anti-nucleopalladation-initiated cascade processes†
Abstract
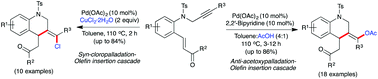
Palladium(II)-catalyzed regioselective syn-chloropalladation and anti-acetoxypalladation-initiated cascade processes were developed for the synthesis of functionalized tetrahydroquinolines. A series of N-propargyl arylamines tethered with an α,β-unsaturated carbonyl scaffold underwent atom economical cascade reactions to deliver chloro- and acetoxy-substituted tetrahydroquinolines bearing an exocyclic double bond in high yields. A mechanism is proposed for these cascade processes involving a sequential syn-chloropalladation or anti-acetoxypalladation of alkynes followed by intramolecular olefin insertion (6-exo-trig) and protonolysis steps. The reaction was completely regioselective and the terminal aryl/alkyl group of the propargyl moiety dictated the regiochemistry of the initial nucleopalladation. The role of the bidentate nitrogen ligand is crucial to trigger the acetoxypalladation-initiated cascade sequence in contrast to the chloropalladation-initiated process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...