Acid-promoted synthesis and photophysical properties of substituted acridine derivatives†
Abstract
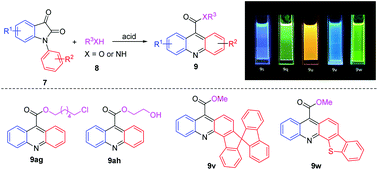
A simple and efficient synthetic protocol for the preparation of acridinium esters and amides through the cyclization and esterification or amidation of isatins with alcohols or amines as nucleophiles in the presence of CF3SO3H is established. A series of polycyclic acridine derivatives bearing large π-conjugated systems were obtained in high yields, including some key intermediates for the synthesis of biologically active molecules. The photophysical properties of these synthesized acridines were investigated, demonstrating that the sulfur heterocyclic acridine 9w was obtained in a high quantum yield.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...