Diastereoselective synthesis and conformational analysis of 4,5-difluoropipecolic acids†
Abstract
Stereoselectively-fluorinated analogs of pipecolic acid have been investigated through a combined theoretical and experimental approach. Three of the four possible diastereoisomers of 4,5-difluoropipecolic acid were successfully synthesized via deoxyfluorination chemistry, navigating a complex reaction network that included neighboring group participation, rearrangement, and elimination pathways. A DFT-based conformational study, supported by NMR J-based analysis, revealed that the different diastereoisomers of 4,5-difluoropipecolic acid preferentially adopt different puckers of the six-membered ring. These findings could have future relevance for the conformational control of biologically active peptides.
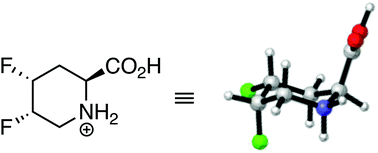
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...