The catalyst-free decarboxylative dearomatization of isoquinolines with β-keto acids and sulfonyl chlorides in water: access to dihydroisoquinoline derivatives †
Abstract
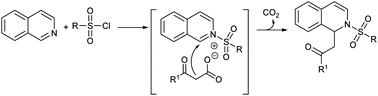
An efficient and concise catalyst-free one-pot synthetic protocol for obtaining dihydroisoquinoline derivatives has been developed via the three-component condensation of isoquinolines with β-keto acids and sulfonyl chlorides. This transformation involving decarboxylative dearomatization worked well under mild and water-mediated conditions. The protocol tolerates diverse functional groups, furnishing the dihydroisoquinoline products in good to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...