On the role of the vinylsulfoxide side chain of dirchromone towards its bioactivities†
Abstract
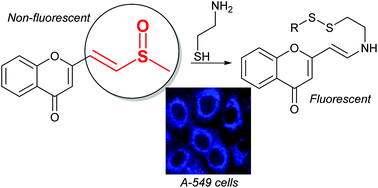
Analogs of dirchromone were prepared to shed light on the pivotal role of its peculiar vinylsulfoxide side chain towards its cytotoxic and antimicrobial properties, especially dependant upon the presence and oxidation state of sulfur. The reaction of dirchromone with cysteamine revealed a surprising Michael acceptor behavior with elimination of the methylsulfinyl moiety and redox transformation of the sulfur atom that could be involved in the mode of action of dirchromone within cells.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...