An optimized protocol for the synthesis of N-2-hydroxybenzyl-cysteine peptide crypto-thioesters†
Abstract
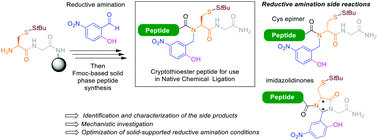
We herein report a robust upgraded synthetic protocol for the synthesis of N-Hnb-Cys crypto-thioester peptides, useful building blocks for segment-based chemical protein synthesis through native chemical ligation. We recently observed the formation of an isomeric co-product when using a different solid support than the originally-reported one, thus hampering the general applicability of the methodology. We undertook a systematic study to characterize this compound and identify the parameters favouring its formation. We show here that epimerization from L- to D-cysteine occurred during the key solid-supported reductive amination step. We also observed the formation of imidazolidinones by-products arising from incomplete reduction of the imine. Structural characterization combined with the deciphering of underlying reaction mechanisms allowed us to optimize conditions that abolished the formation of all these side-products.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...