Syntheses and reactivity of spiro-epoxy/aziridine oxindole cores: developments in the past decade
Abstract
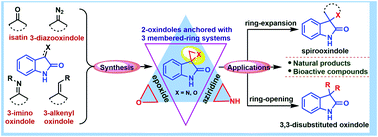
Spiro-epoxy/aziridine oxindole frameworks are considered as an efficient structural toolbox to obtain C3-functionalised oxindole derivatives. These 3,3′-spiro-cyclic precursors are highly susceptible towards nucleophiles owing to their inherent ring strain. Their versatile reactivity has opened many potential synthetic transformations, allowing access to bioactive molecules as well as natural products. The present review aims to elaborate various enabling strategies applied in the successful synthesis of strained spiro-cyclic oxindole scaffolds. Furthermore, the nucleophilic ring-opening/expansion and cycloaddition reactions of spiro-epoxy/aziridine oxindole are well discussed. Moreover, mechanistic insights to define the regio- and stereo-chemical outcome of the products have also been highlighted briefly.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...