A versatile approach to the synthesis of mannosamine glycosides†
Abstract
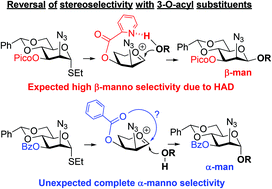
O-Picoloyl protecting groups at remote positions can affect the stereoselectivity of glycosylation by means of the H-bond-mediated aglycone delivery (HAD) pathway. A new practical method for the stereoselective synthesis of β-glycosides of mannosamine is reported. The presence of the O-picoloyl group at the C-3 position of a mannosamine donor can provide high or complete stereocontrol. The method was also utilized for the synthesis of a biologically relevant trisaccharide related to the capsular polysaccharide of Streptococcus pneumoniae serotype 4. Also reported herein is a method to achieve complete α-manno stereoselectivity with mannosamine donors equipped with 3-O-benzoyl group.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...