Palladium-catalyzed double coupling reaction of terminal alkynes with isocyanides: a direct approach to symmetrical N-aryl dialkynylimines†
Abstract
A novel and efficient one-pot synthesis of symmetrical N-aryl dialkynylimines via palladium-catalyzed and copper-promoted isocyanide insertion, cross-coupling and elimination has been developed. This method features readily available starting materials, mild reaction conditions and high atom efficiency as well as simple one-pot operation, which make this strategy highly attractive. Moreover, 2-iodobenzo[f]quinoline derivatives can be obtained via electrophilic cyclization of N-aryl dialkynylimines.
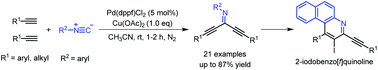


 Please wait while we load your content...
Please wait while we load your content...