Carbazole modified oligonucleotides: synthesis, hybridization studies and fluorescence properties†
Abstract
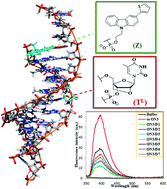
Synthesis of the novel thiophenyl carbazole phosphoramidite DNA building block 5 was accomplished in four steps using a Suzuki–Miyaura cross-coupling reaction from the core carbazole and it was seamlessly accommodated into a 9-mer DNA-based oligonucleotide by incorporation at the flanking 5′-end in combination with a central insertion of an LNA-T nucleotide. The carbazole-containing oligonucleotide was combined in different duplex hybrids, which were characterized by thermal denaturation, circular dichroism and fluorescence studies. The carbazole monomer modulates the duplex stability in various ways. Thus, monomer Z increased the thermal stability of the 9-mer towards the complementary 9-mer/15-mer DNA duplex by 4.2 °C. Furthermore, indications of its intercalation into the duplex were obtained by modeling studies and robust decreases in fluorescence emission intensities upon duplex formation. In contrast, no clear intercalating tendency was corroborated for monomer Z within the DNA/RNA hybrid duplex as indicated by moderate quenching of the fluorescence and similar duplex thermal stabilities relative to the corresponding control duplex. The recognition efficiencies of the carbazole modified oligonucleotide toward single nucleotide mismatches were studied with two 15-mer model targets (DNA and RNA). For both systems, mismatches positioned at the juxtaposition of the carbazole monomer showed pronounced deceases in thermal denaturation temperature. Steady-state fluorescence emission studies of all mismatched duplexes with incorporation of Z monomer typically displayed efficient fluorescence quenching.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...