Yulei Zhao, Xuqiang Guo, Yulan Du, Xinrui Shi, Shina Yan, Yunlin Liu and Jinmao You
Org. Biomol. Chem., 2020,18, 6881-6888
DOI:
10.1039/D0OB01504K,
Paper
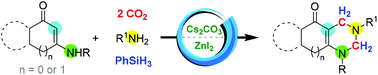
A methylenation–cyclization reaction, employing cyclic enaminones with primary aromatic amines and two molecules of CO2, furnishing fused-tetrahydropyrimidines, is discussed. In this Cs2CO3 and ZnI2 catalyzed one-pot two-step procedure, two molecules of CO2 were selectively converted to methylene groups. The multi-component reaction might proceed through the formation of bis(silyl)acetal which was followed by condensation and further aza-Diels–Alder reaction. Hydroquinazoline, hydrocyclopenta[d]pyrimidine and hydroindeno[1,2-d]pyrimidine derivatives could be prepared with CO2 as the C1 source, effectively.