Photoinduced iodine-mediated tandem dehydrogenative Povarov cyclisation/C–H oxygenation reactions†
Abstract
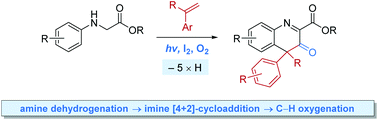
We report metal-free, photoinduced aerobic tandem dehydrogenative Povarov cyclisation/Csp3–H oxygenation reactions between N-aryl glycine esters and α-substituted styrenes, which efficiently lead to 4,4-disubstituted dihydroquinoline-3-ones under mild conditions. The reactions are mediated by iodine along with visible light irradiation, which allows for the in situ generation of the essential Brønsted acid HI, to catalyse the key imine [4+2]-cycloaddition.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...