Deprotection of S-acetamidomethyl cysteine with copper(ii) and 1,2-aminothiols under aerobic conditions†
Abstract
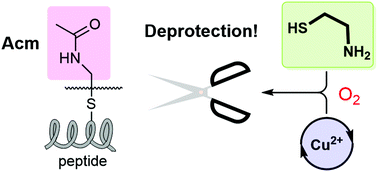
Ring-opening by CuSO4 of a 1,3-thiazolidine carbonyl structure (Thz) as an N-terminal cysteine (Cys) residue revealed that an intramolecular S-acetamidomethyl cysteine (Cys(Acm)) can also be deprotected with concomitant formation of a disulphide bond connecting the two Cys residues. A mechanistic study on the disulphide formation led to a general protocol for deprotection of the S-Acm group by CuSO4 and a 1,2-aminothiol under aerobic conditions. Application of this new deprotection reaction allowed for the synthesis of Apamin, a peptide with two-disulphides in a one-pot/stepwise disulphide-bridging procedure.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...