Iron-catalyzed radical cascade 6-endo cyclization of dienes towards fused nitrogen heterocycles initiated by an alkoxycarbonyl radical†
Abstract
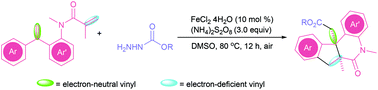
An iron-catalyzed radical cascade cyclization of dienes initiated by an alkoxycarbonyl radical has been developed in the presence of (NH4)2S2O8, leading to a series of fused nitrogen heterocyclic compounds under relatively mild reaction conditions. The reaction is triggered by the addition of an alkyoxycarbonyl radical derived from the cleavage of alkoxyformyl hydrazide. Afterward, the formed nucleophilic radical preferred addition to the electron-neutral vinyl rather than the electron-deficient vinyl, followed by cascade 6-endo cyclization and further radical cyclization.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...