Exploring the behavior of the NFSI reagent as a nitrogen source
Abstract
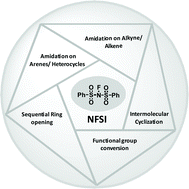
The diverse biological activities of nitrogen-containing compounds make the construction of the C–N bond of great importance. As N-fluorobenzenesulfonimide, one of the most abundant chemical feedstock, has a dual behaviour, i.e. as an electrophilic fluorination and amidation source, it attracts the attention of synthetic chemists for exploitation. This review comprehensively summarizes the significant progress of the efficient and mild amidation reactions, with an emphasis on approaches for the generation of nitrogen-centered intermediates, related mechanisms and new synthetic chemistry methods that offer opportunities to overcome obstacles in pharmaceutical applications. In this perspective, we discuss the developments in the amidation reaction using NFSI in the past decade. We discuss the recent progress, challenges and future outcomes in the area of amidation chemistry using commercially available NFSI.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...