BINOL derivatives-catalysed enantioselective allylboration of isatins: application to the synthesis of (R)-chimonamidine†
Abstract
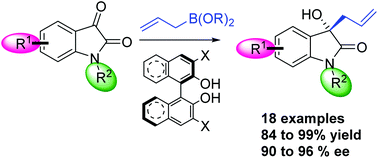
The asymmetric synthesis of the 3-allyl-3-hydroxyoxindole skeleton was accomplished in yields up to 99% via a metal-free and enantioselective allylation of isatins (90–96% ee) using BINOL derivatives as catalysts and an optimized allylboronate. This methodology was applied at a gram-scale to the synthesis of the natural product (R)-chimonamidine.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...