Improved water solubility and photodynamic activity of hydroxy-modified porphyrins by complexation with cyclodextrin†
Abstract
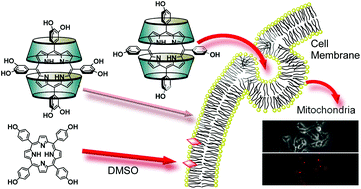
Porphyrin derivatives with hydroxyphenyl or dihydroxyphenyl moieties in the meso-position were able to dissolve in water by complexation with two trimethyl-β-cyclodextrins (TMe-β-CDxs) without further chemical modification of porphyrins or addition of dimethyl sulfoxide as a co-solvent. TMe-β-CDx-complexed tetra(hydroxyphenyl)porphyrins (THPPs) with phenols in meso-positions have a much higher photodynamic activity than TMe-β-CDx-complexed tetrakis(dihydroxyphenyl)porphyrins (TDHPPs) with dihydroxyphenyl in the meso-position. The main reason for the different photoactivity is due to the intracellular uptakes of these complexes. Thus phenols in meso-positions of porphyrin derivatives in the complexes were recognized by HeLa cell receptors. Furthermore, the photodynamic activity of TMe-β-CDx-complexed THPP was much higher than that of THPP injected as a dimethyl sulfoxide solution.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...