Synthesis of cyclopentaquinolinone and cyclopentapyridinone from ortho-alkynyl-N-arylaldehyde via superbase-promoted C–N, C–O and C–C bond formation†
Abstract
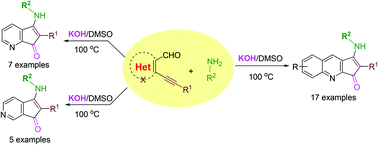
An environmentally benign, transition metal-free, superbase-mediated intramolecular annulation of o-alkynylaldehydes with primary amines forms highly functionalized amino-substituted cyclopentaquinolinones and cyclopentapyridinones via C–N, C–C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond formation. Contrary to the traditional approaches of ring closures, a different mode of annulation is disclosed. The protocol involves the in situ generations of imine intermediate followed by potassium hydroxide-promoted intramolecular cyclization and subsequent dimethyl sulfoxide induced dehydrogenation leads to the formation of N-heterocycles. X-ray crystallographic studies support the assigned structures of the amino-fused N-heterocycles.
O bond formation. Contrary to the traditional approaches of ring closures, a different mode of annulation is disclosed. The protocol involves the in situ generations of imine intermediate followed by potassium hydroxide-promoted intramolecular cyclization and subsequent dimethyl sulfoxide induced dehydrogenation leads to the formation of N-heterocycles. X-ray crystallographic studies support the assigned structures of the amino-fused N-heterocycles.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...