Nickel/briphos-catalyzed transamidation of unactivated tertiary amides†
Abstract
The transamidation of tertiary amides was achieved via nickel catalysis in combination with briphos ligands. N-Methyl-N-phenylbenzamide derivatives reacted with primary amines in the presence of NiCl2/briphos L4 to provide the transamidated products in moderate to good yields. Primary aromatic amines delivered higher product yields than aliphatic amines.
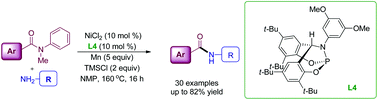
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...