Dual-binding conjugates of diaromatic guanidines and porphyrins for recognition of G-quadruplexes†
Abstract
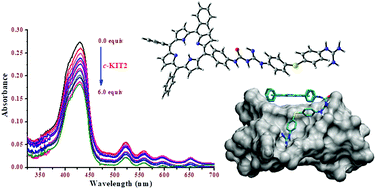
The first conceptualised class of dual-binding guanine quadruplex binders has been designed, synthesised and biophysically studied. These compounds combine diaromatic guanidinium systems and neutral tetra-phenylporphyrins (classical binding moiety for guanine quadruplexes) by means of a semi-rigid linker. An extensive screening of a variety of guanine quadruplex structures and double stranded DNA via UV-vis, FRET and CD experiments revealed the preference of the conjugates towards guanine quadruplexes. Additionally, docking studies indicate the potential dual mode of binding.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...