Photocatalytic dual decarboxylative alkenylation mediated by triphenylphosphine and sodium iodide†
Abstract
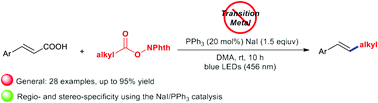
An efficient photocatalytic dual decarboxylative alkenylation of α,β-unsaturated carboxylic acids and alkyl N-hydroxyphthalimide (NHP) esters mediated by triphenylphosphine and sodium iodide has been developed. This protocol proceeds under 456-nanometer irradiation by visible blue light in the absence of transition metals or organic dye based photoredox catalysts. The reaction is successfully applied to a wide range of redox-active esters derived from aliphatic carboxylic acids (1°, 2° and 3°) and α-amino acids, enabling transformations of diverse α,β-unsaturated carboxylic acids to α,β-alkylated styrenes with high efficiency and excellent selectivity under mild conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...