Synthetic studies on palmerolide C: synthesis of an advanced intermediate towards the revised structure of palmerolide C†
Abstract
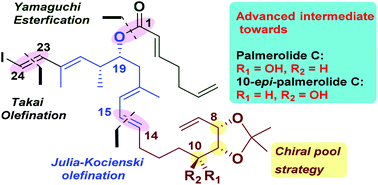
A stereoselective synthesis of the highly advanced intermediates towards the revised structure of palmerolide C and 10-epi-palmerolide C is described in this paper. The required key fragments C1–C6, C7–C14 and C15–C23 have been successfully assembled in a convergent manner to access the C1–C23 framework bearing all the five stereocenters present in the natural product. The synthesis involves the Julia–Kocienski reaction, Yamaguchi esterification, Takai olefination and regioselective epoxide opening as key steps. The proposed route is flexible and could also be applied to the synthesis of structurally related palmerolides.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...