Catalytic C3 aza-alkylation of indoles
Abstract
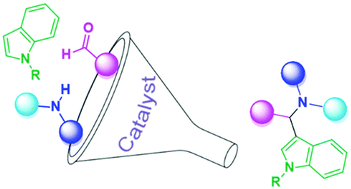
The aza-alkylation reaction at indole's C3 position allows the introduction of a differently substituted aminomethyl group, with the formation of a new stereogenic centre. The reaction involves essentially three different partners: an indole, aldehyde and amine. The formation of the reactive iminium species can be catalyzed by metals, Brønsted acids, Lewis acids or organocatalysts. The stereoselective reaction is feasible with satisfactory outcomes. This review summarizes the recent (2000–2019) meaningful papers in which the in-depth study and exploitation of this reactivity are reported.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...