Palladium-catalyzed synthesis of 5-amino-1,2,4-oxadiazoles via isocyanide insertion†
Abstract
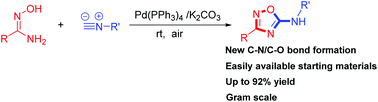
A convenient and efficient palladium-catalyzed approach has been developed for the synthesis of 5-amino-1,2,4-oxadiazoles from amidoximes and isocyanides. Various 5-amino-1,2,4-oxadiazoles were obtained in moderate to high yields under mild conditions. The key to the success of this strategy involves new C–N bond and C–O bond formation via palladium-catalyzed isocyanide insertion.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...