Copper(i)-catalyzed intermolecular cyanoarylation of alkenes: convenient access to α-alkylated arylacetonitriles†
Abstract
A novel Cu(I)-catalyzed intermolecular cyanoarylation of alkenes with diaryliodonium salts as a radical arylating reagent and tetra-butylammonium cyanide as an electrophilic cyanating reagent was established. A broad range of α-alkylated arylacetonitriles were efficiently constructed in good to excellent yields under base- and oxidant-free and mild conditions.
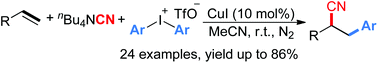
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...