Construction of chiral chroman scaffolds via catalytic asymmetric (4 + 2) cyclizations of para-quinone methide derivatives with 3-vinylindoles†
Abstract
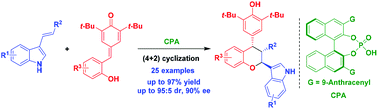
A catalytic asymmetric (4 + 2) cyclization of ortho-hydroxyphenyl-substituted para-quinone methide derivatives with 3-vinylindoles has been established in the presence of chiral phosphoric acid, which provides a series of chiral chroman derivatives bearing an indole moiety in generally high yields (up to 97%), and with good enantioselectivities (up to 90% ee) and moderate to good diastereoselectivities (up to 95 : 5 dr). This reaction has not only fulfilled the need of developing catalytic asymmetric (4 + 2) cyclizations of para-quinone methide derivatives with electron-rich alkenes, but also provided a useful method for constructing chiral chroman scaffolds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...