Design of potent ABA receptor antagonists based on a conformational restriction approach†
Abstract
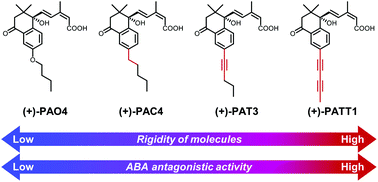
The physiological functions of the plant hormone abscisic acid (ABA) are triggered by interactions between PYR/PYL/RCAR receptors (PYLs) and group-A protein phosphatases 2C (PP2Cs). PYL agonists/antagonists capable of inducing/disrupting these interactions would be valuable in investigating the regulatory mechanisms of ABA signaling. Previously, we developed (+)-PAO4, a high-affinity PYL antagonist, by conformationally restricting the S-hexyl chain of our first reported PYL antagonist, 3′-hexylsulfanyl-ABA. Although (+)-PAO4 shows a greater binding affinity for Arabidopsis PYL5 compared with 3′-hexylsulfanyl-ABA, it is not able to completely block the ABA responses both in vitro and in vivo. Therefore, we designed novel conformationally restricted PYL antagonists in which the O-butyl chain of (+)-PAO4 was replaced with a pentyl (PAC4), a pentyne (PAT3) or a pentadiyne (PATT1) chain. (+)-PAT3 and (+)-PATT1 suppressed the ABA-induced inhibition of Arabidopsis seed germination more strongly than (+)-PAO4, but contrary to expectations, the affinity of each compound for PYL5 was almost the same as that of (+)-PAO4. Subsequent biochemical analyses revealed that unlike (+)-PAO4, (+)-PAT3 and (+)-PATT1 completely abolished ABA-induced PYL–PP2C interactions without partial agonistic activities. The superior PYL antagonist functions of (+)-PAT3 and (+)-PATT1 over (+)-PAO4 may explain their potent antagonistic activities against exogenous ABA in vivo. Furthermore, (+)-PAT3 and (+)-PATT1 also suppressed ABA responses in rice, indicating that both compounds are useful chemical tools for ABA-signaling studies, not only in dicots but also in monocots.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...