Monophenolase and catecholase activity of Aspergillus oryzae catechol oxidase: insights from hybrid QM/MM calculations†
Abstract
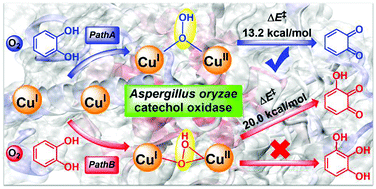
Catechol oxidase from Aspergillus oryzae (AoCO4) can not only catalyze oxidation of o-diphenols to o-quinones, but can also catalyze monooxygenation of small phenolics. To gain insight into the catecholase and monophenolase activities of AoCO4, the reaction mechanism of catechol oxidation was investigated by means of hybrid quantum mechanical/molecular mechanical (QM/MM) calculations. The oxy-form of AoCO4 was found to be a μ–η2:η2 side-on peroxo dicopper(II) complex, which can undergo a proton coupled electron transfer from the substrate rather than a proton transfer from the nearby Ser302 residue to generate a hydroperoxide. The μ-1,1-OOH Cu2(I,II) complex is thermodynamically more stable than the μ–η1:η2 hydroperoxide. Moreover, the cleavage of the O–O bond in the μ-1,1-OOH Cu2(I,II) intermediate has a much lower barrier than that in the μ–η1:η2 hydroperoxide species. In both cases, the O–O bond cleavage is the rate-limiting step, generating the reactive (μ-O˙)(μ-OH) dicopper(II) complex. In addition, our results demonstrated that the oxidation of catechol to quinone is much more preferred than the hydroxylation reaction. These findings may provide useful information for understanding the reactivity of the Cu2O2 active site of coupled binuclear copper enzymes.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...