Remote azidation of C(sp3)–H bonds to synthesize δ-azido sulfonamides via iron-catalyzed radical relay†
Abstract
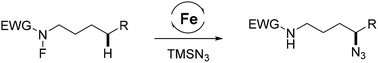
With an iron catalyst playing dual roles as a radical initiator and terminator, we report a selective remote C–H functionalization to access δ-azido sulfonamides through a radical relay process. The reaction of N-fluorosulfonamide furnishes the corresponding products in excellent yields with high regioselective control. The key to success is the highly efficient iron-mediated redox azido transfer to the in situ generated carbon radical. The products provide incentives for drug discovery and ligand designs.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...