Total synthesis and biological evaluation of seven new anti-inflammatory oxacyclododecindione-type macrolactones†
Abstract
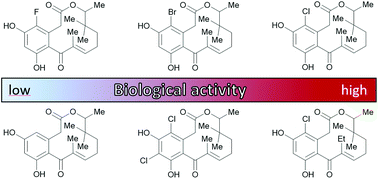
Through variation of our previously published total synthesis of two highly active anti-inflammatory macrolactones from the oxacyclododecindione family (J. Tauber, M. Rohr, T. Walter, G. Erkel and T. Opatz, Org. Biomol. Chem., 2015, 13, 7813–7821), seven new representatives of this compound class were prepared. Substitution of the 14-hydroxy group in oxacyclododecindione with a methyl substituent provided a readily accessible non-natural analogue which has similar pharmacological properties to the scarcely available natural product. Since the producible amount of substance is therefore no longer restricted by low fermentation yields, extensive in vivo studies become possible for the first time. Based on this finding, further investigations on structure–activity relationships were undertaken by variation of the halogen atom, which showed that exchange or omission of the chloro substituent led to significantly lower binding affinities. Furthermore, it was found that elongation of the crucial and characteristic aliphatic side chain at C-10 also increased the IC50 value in the biological assays of interest.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...