Differential scanning fluorimetry (DSF) screen to identify inhibitors of Hsp60 protein–protein interactions†
Abstract
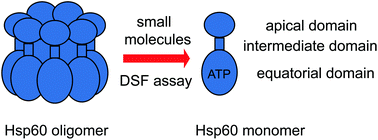
There are relatively few methods available for discovering inhibitors of the protein–protein interactions (PPIs) that hold together homo-oligomers. We envisioned that Differential Scanning Fluorimetry (DSF) might be a versatile way to discover this type of inhibitor because oligomers are often more thermally stable than monomers. Using the homo-heptameric chaperonin, Hsp60, as a model, we screened ∼5000 diverse compounds in 384-well plates by DSF, revealing molecules that partially inhibited oligomerization. Because DSF does not require protein labeling or structural information, we propose that it could be a versatile way to uncover PPI inhibitors.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...