A fully synthetic 6-aza-artemisinin bearing an amphiphilic chain generates aggregates and exhibits anti-cancer activities†
Abstract
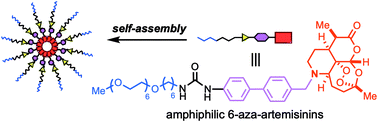
Installation of a nitrogen at the C6 position of artemisinin facilitates the addition of a functional unit on the cyclohexane moiety (C-ring). In this study, conjugation of an amphiphilic chain, composed of sequentially connected hydrophilic oligoethylene glycol, hydrophobic alkyl chain, urea, and 4,4′-disubstituted biphenyl linker, imparted self-assembling properties. The fully synthetic mid-molecular weight 6-aza-artemisinin 6 bearing the amphiphilic moiety formed aggregates (approx. 200 nm) at ambient temperature and exhibited increased in vitro anti-cancer activities compared to the N-benzylated aza-artemisinin 5.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...