Stereoselective synthesis of 2,6-disubstituted piperidine alkaloids
Abstract
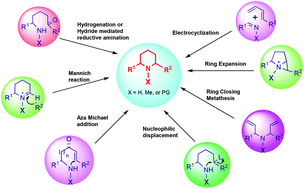
Among the large number of structurally diverse alkaloids, 2,6-disubstituted piperidine and its analogs have often been targeted when exploiting new synthetic techniques perhaps because of their strong pharmacological properties. This review outlines synthetic strategies to build the 2,6-disubstituted piperidine structural motif with a focus on stereochemical control of two substituents at C2 and C6. The key reactions in this process are then classified on the basis of how the piperidine rings were built with specific examples of natural products that control the stereochemical outcomes and their transition states.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...