Isatin as a 2-aminobenzaldehyde surrogate: transition metal-free efficient synthesis of 2-(2′-aminophenyl)benzothiazole derivatives†
Abstract
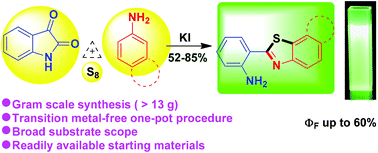
A transition metal-free, convenient, and efficient practical approach has been devised for the synthesis of substituted 2-(2′-aminophenyl)benzothiazoles via a sulfur insertion strategy using isatin derivatives as 2-aminobenzaldehyde surrogates. KI assisted one-pot operation of isatin, arylamines and elemental sulfur resulted in the formation of a C–N and two C–S bonds and cascade cleavage of the isatin ring resulting in the formation of 2-(2′-aminophenyl)benzothiazoles. The significant features of this strategy are the readily available and inexpensive starting materials, broad substrate scope, sustainable reaction conditions and high yield of products. Importantly, the strategy was found to be appropriate for gram scale synthesis (>10 g) of 2-(2′-aminophenyl)benzothiazole derivatives. Moreover, the excellent photophysical properties (ΦF up to 60%) of 2-(2′-aminophenyl)benzothiazole derivatives provide huge scope in materials science.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...