Practical direct synthesis of N-aryl-substituted azacycles from N-alkyl protected arylamines using TiCl4 and DBU†
Abstract
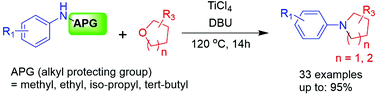
A novel transformation of N-alkyl protected arylamines and cyclic ethers into N-aryl substituted azacycles is described. Alkyl groups have been used for the protection of amines in organic syntheses. In this synthesis, N-alkyl protected arylamines were reacted with cyclic ethers in the presence of TiCl4 and DBU, crucial reagents affording five- and six-membered azacycles. In particular, utilization of the novel TiCl4/DBU-mediated reaction allows various N-alkyl protected arylamines such as N-methyl-, N-ethyl-, N-isopropyl, and N-tert-butyl arylamines to be readily converted into N-aryl substituted azacycles in high yields. This practical approach using various N-alkyl arylamines leads to the efficient preparation of azacycles.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...