Total synthesis of four stereoisomers of methyl 4,8,12-trimethylpentadecanoate, a major component of the sex pheromone of the stink bug Edessa meditabunda†
Abstract
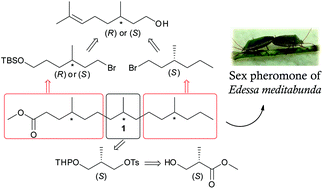
The male-produced sex pheromone of the stink bug Edessa meditabunda was previously identified as a mixture of the esters methyl 4,8,12-trimethylpentadecanoate (1) and methyl 4,8,12-trimethyltetradecanoate (2), produced in a ratio of 92 : 8, respectively. Bioassays showed that the synthetic major compound alone is sufficient to elicit a response from females, and that it is as attractive as the natural extract. Here we present a stereoselective synthesis of four stereoisomers of methyl 4,8,12-trimethylpentadecanoate. The synthetic route was based on the connection of three chiral building blocks. High stereoisomeric purity was achieved by using commercially available compounds with defined stereochemistry, (R) and (S)-citronellol and methyl (S)-3-hydroxy-2-methylpropionate. Different stereoisomers were synthesized by swapping the sequence in which the building blocks were inserted into the synthetic route. The key steps in the synthesis were coupling reactions using the Fouquet–Schlosser variant of the Grignard reaction. Although the absolute configuration of the natural product remained elusive due to chromatographic inseparability of the stereoisomers, the syntheses gave access to both enantiomers of the biosynthetically most likely stereoisomer syn,syn-1, while all other stereoisomers can be efficiently synthesized by our straightforward approach.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...