Disulphide-mediated site-directed modification of proteins†
Abstract
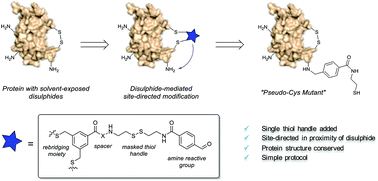
Methods for chemical modification of native proteins in a controlled fashion are in high demand. Here, a novel protocol that exploits bifunctional reagents for transient targeting of solvent exposed disulphides to direct the introduction of a single exogenous reactive thiol handle at a lysine side chain has been developed. The protocol has successfully been applied to functionalize six different Fabs and human growth hormone.
- This article is part of the themed collections: Editor’s Collection, Chemical Biology in OBC and Methodology development for protein modifications


 Please wait while we load your content...
Please wait while we load your content...