Cu-Photoredox-catalyzed C(sp)–C(sp3) coupling of redox-active esters with terminal alkynes†
Abstract
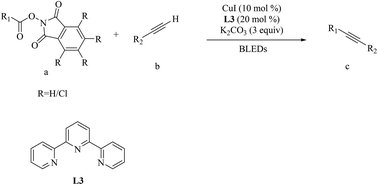
Visible-light-induced C(sp)–C(sp3) coupling of redox-active esters with terminal alkynes has been developed. The activation of carboxylic acids as their redox-active ester derivatives was important for this decarboxylative alkynylation. The strategy established here facilitates the straightforward introduction of triple-bonded functional groups and avoids additional photocatalysts. A wide range of primary, secondary and tertiary acids can be converted into the target products; so this reaction exhibits a broad substrate scope and tolerance of functional groups. Mechanistic experiments suggested that this reaction may undergo a radical process. Under mild reaction conditions, a copper acetylide ligand as a photocatalyst delivered an electron to redox-active ester derivatives, and generated alkyl radicals. The radicals reacted with Cu(II) to deliver a Cu(III) complex, and then reductive elimination gave the products.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...