Synthesis of biaryl ketones by arylation of Weinreb amides with functionalized Grignard reagents under thermodynamic control vs. kinetic control of N,N-Boc2-amides†
Abstract
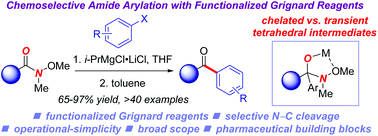
A highly efficient method for chemoselective synthesis of biaryl ketones by arylation of Weinreb amides (N-methoxy-N-methylamides) with functionalized Grignard reagents is reported. This protocol offers rapid entry to functionalized biaryl ketones after Mg/halide exchange with i-PrMgCl·LiCl under operationally-simple and practical reaction conditions. The scope of the method is highlighted in >40 examples, including bioactive compounds and pharmaceutical derivatives. Collectively, this transition-metal-free approach offers a major advantage over the recently established cross-coupling of amides by oxidative addition of N–C(O) bonds. Considering the utility of amide acylation reactions in modern synthesis, we expect that this method will be of broad interest.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...