Sulfur-mediated synthesis of unsymmetrically substituted N-aryl oxalamides by the cascade thioamidation/cyclocondensation and hydrolysis reaction†
Abstract
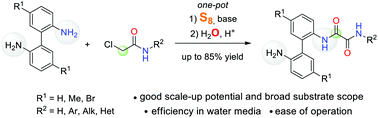
A facile and straightforward synthesis of unsymmetrically substituted N-aryl oxalamides from 2,2′-biphenyldiamines, 2-chloroacetic acid derivatives, elemental sulfur, and water has been developed. This protocol is distinguished by efficiency in water under metal-free conditions for N-aryl oxalamides bearing a side-chain NH2-group; it can be adapted for scale-up synthesis. The scope and limitations of this transformation have been investigated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...