One-pot synthesis of β-O-4 lignin models via the insertion of stable 2-diazo-1,3-dicarbonyls into O–H bonds†
Abstract
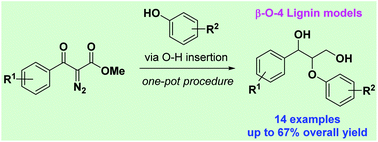
Because lignin is a macromolecule that is a sustainable source of aromatic compounds, model substrates are commonly used to increase our understanding of its complex structure. However, few methods have been described for the synthesis of these models. Herein, we describe a new route towards the synthesis of β-O-4 lignin models by intermolecular O–H insertion reactions with simple and stable diazocarbonyls. The benefits of this developed method were shorter reaction times and high yields, as well as mild and environmentally friendly conditions.


 Please wait while we load your content...
Please wait while we load your content...