C4–C5 fused pyrazol-3-amines: when the degree of unsaturation and electronic characteristics of the fused ring controls regioselectivity in Ullmann and acylation reactions†
Abstract
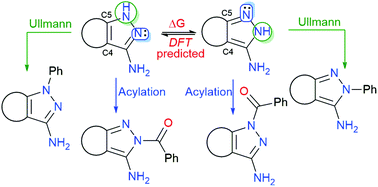
Pyrazol-3-amine is a scaffold present in a large number of compounds with a wide range of biological activities and, in many cases, the heterocycle is C4–C5 fused to a second ring. Among the different reactions used for the decoration of the pyrazole ring, Ullmann and acylation have been widely applied. However, there is some confusion in the literature regarding the regioselectivity of such reactions (substitution at N1 or N2 of the pyrazole ring) and no predictive rule has been so far established. As a part of our work on 3-amino-pyrazolo[3,4-b]pyridones 13, we have studied the regioselectivity of such reactions in different C4–C5 fused pyrazol-3-amines. As a rule of thumb, the Ullmann and acylation reactions take place, predominantly, at the NH and non-protonated nitrogen atom of the pyrazole ring respectively, of the most stable initial tautomer (1H- or 2H-pyrazole), which can be easily predicted by using DFT calculations.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...