Acylation of oxindoles using methyl/phenyl esters via the mixed Claisen condensation – an access to 3-alkylideneoxindoles†
Abstract
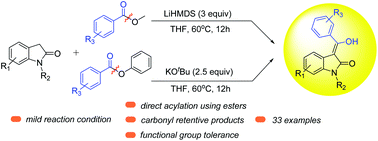
Predominantly, aggressive acid chlorides and stoichiometric coupling reagents are employed in the acylating process for synthesizing carbonyl tethered heterocycles. Herein, we report simple acyl sources, viz. methyl and phenyl esters, which acylate oxindoles via the mixed Claisen condensation. This straightforward protocol is mediated by LiHMDS and KOtBu and successfully applied to a wide range of substrates. It is a noteworthy transformation that skips the stepwise generation of enolates and acylation, and the reaction is performed at a moderate temperature with no side reactions. This protocol produces the first examples of ortho-substituents in an aryl ring flanked with electron-donating and electron-withdrawing substrates. Interestingly, robust organometallic ferrocenyl methyl ester cleaved under these conditions with ease. Furthermore, biologically important Tenidap's analog was synthesized by this protocol.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...