Doubly vinylogous and doubly rearomative functionalization of 2-alkyl-3-furfurals†
Abstract
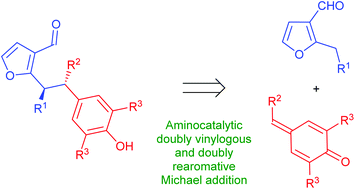
The manuscript describes a straightforward functionalization of 2-alkyl-3-furfurals via simple aminocatalytic conjugate addition. The reaction proceeds through the formation of dearomatized dienamine-like intermediate that undergoes 1,6-addition to 4-alkylidene-2,6-dialkylcyclohexa-2,5-dienones. This process can be described as doubly rearomative as it proceeds with the re-formation of both furan and phenyl aromatic moieties. Target products have been obtained in a highly stereoselective manner, providing an interesting example of 2-alkyl-3-furfural functionalization via doubly vinylogous Michael addition. The mechanism of the reaction has been studied by means of computational methods.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...