Gold(iii)-catalyzed azide-yne cyclization/O–H insertion cascade reaction for the expeditious construction of 3-alkoxy-4-quinolinone frameworks†
Abstract
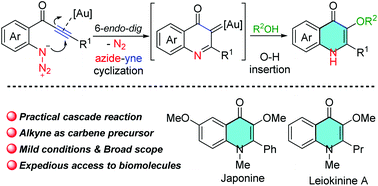
A gold-catalyzed 6-endo-dig azide-yne cyclization/O–H insertion cascade reaction of azide-tethered alkynes with alcohols has been developed, and it provides an expeditious access to 3-alkoxy-4-quinoline derivatives in good to high yields under mild and neutral reaction conditions with broad substrate generality. The utility of this method is emphasized by a scalable experiment and concise total synthesis of a bioactive natural product Leiokinine A, and other bioactive quinoline analogs.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...