Catalyst- and solvent-free efficient access to N-alkylated amines via reductive amination using HBpin†
Abstract
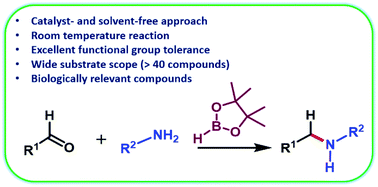
A sustainable approach which works under catalyst- and solvent-free conditions for the synthesis of structurally diverse secondary amines has been uncovered. This one-pot protocol works efficiently at room temperature and is compatible with a wide range of sterically and electronically diverse aldehydes and primary amines. Notably, this simple process offers scalability, excellent functional group tolerance, chemoselectivity, and is also effective at the synthesis of biologically relevant molecules.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...